Light-powered phagobots: dual-mode optical control of living immune microrobots
FAYETTEVILLE, GA, UNITED STATES, May 27, 2025 /EINPresswire.com/ -- Immunomodulation based on macrophages shows great potential in tackling major diseases such as infections and cancers. However, precision control of these cells in vivo has proven challenging. To solve this problem, researchers from China developed a dual-modal optical strategy to turn macrophages into “phagobot” that can seek and eliminate bio-threats on command both in vitro and in vivo. Without genetic modification or synthetic materials, this non-genetic living immune microrobot holds great potential for precision immunotherapy.
Macrophages and other immune cells are the natural frontline immune warriors of our body, defending the body against invading pathogens and cancer cells. Yet despite their innate “combat” capabilities, precisely directing and activating these cells in vivo has remained challenging. In recent years, the emergence of bio-microrobots has shown great potential to convert these “natural soldiers” into controllable function units using external physical fields and biomimetic design. However, existing approaches typically rely on magnetic, acoustic or optical fields to drive cell movement, but hard to tuning their biological functions. Besides, these methods require exogenous material or genetic modification, raising serious biosafety and immune-rejection concerns.
In a new paper published in Light: Science & Applications, a team of scientists led by Professor Hongbao Xin from Jinan University, China, has developed a light-powered phagocytic macrophage microrobot (“Phagobot”) that combines innate immune functions with robotic controllability. The phagobot can be “woken up” and navigated simply using a tightly focused near-infrared (NIR) light beam.
“We wanted to find a way to control immune cells with the same precision as machines, but without taking away their natural strengths,” said Professor Xin, the study’s corresponding author. “With this dual-mode optical control strategy, macrophages remain completely natural, yet they can be precisely instructed to move, seek, and phagocytosis of bio-threats both in vitro and in vivo.”
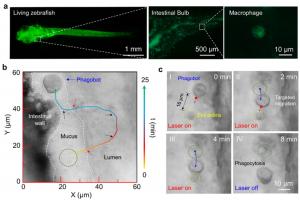
The process begins with a tightly focused NIR laser beam (1064 nm) focusing on the surface of resting macrophages. Within a few minutes, the localized photothermal effect triggers temperature-sensitive ion channels in the cell membrane, causing calcium to rush into the cell. This chain reaction activates the cell’s energy metabolism and leads to a burst of reactive oxygen species (ROS), which is a hallmark of macrophage activation. As a result, the macrophage transforms, extending flexible “arms” called pseudopodia, ready for action.
“It’s like flipping a biological switch with light,” said Xing Li, the paper’s first author and PhD student at Jinan University. “The light doesn’t just move the cell. It turns the cell into a warrior.”
The extended pseudopodia in activated macrophage can act like tiny antennae to sense changes in the extracellular microenvironment. Researchers used gentle optical forces to manipulate the pseudopodia, enabling precise directional control. In this way, the phagobot could be navigated toward target locations with high spatial accuracy.
“Other bio-microrobots that rely on magnetic or acoustic fields to push entire cells, which may inevitably disturb cell activity and immune state. On contrast, this method works at the subcellular level, guiding only the pseudopodia. This keeps the rest of the cell undisturbed, mimicking how immune cells naturally migrate in tissue.” Said the co-corresponding author, Associate Professor Ting Pan.
In laboratory tests, the phagobot showed remarkable efficiency in targeting and engulfing a variety of bio-threats, including Staphylococcus aureus, yeast cells, plastic nanoparticles, and tumor cell debris. The system also proved its power in vivo. Using zebrafish models, researchers labeled macrophages with fluorescent markers and successfully activated and navigated them in the complex, constantly moving gut environment. There, the phagobots located and cleared cellular debris without causing any visible tissue damage even after prolonged light exposure.
“This approach overcomes the two major bottlenecks in the field of bio-microrobots: external driving systems can only drive the cells to move and the need for synthetic or genetic modifications. It provides a non-genetic platform for in vivo immune intervention, offering promising applications in targeted therapy and precision immunomodulation,” these scientists summarized.
References
DOI
10.1038/s41377-025-01881-3
Original Source URL
https://doi.org/10.1038/s41377-025-01881-3
Funding Information
This work was supported by the National Natural Science Foundation of China (12374286, 32271405, 12204196), Guangdong Basic and Applied Basic Research Foundation (2024A1515012514), and the Fundamental Research Funds for the Central Universities (21624217).
Lucy Wang
BioDesign Research
email us here
Distribution channels: Science, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release